Heart disease affects 10 percent of dogs in general practice (Keene et al., 2019), with myxomatous mitral valve disease (MMVD) and dilated cardiomyopathy (DCM) being the most common types of acquired heart disease. MMVD accounts for approximately 75 percent of acquired canine heart disease cases. The highest incidence of MMVD occurs in older, small- to medium-sized dogs weighing less than 20 kilograms. The cause of MMVD remains unknown, but both the disease and its severity may have a genetic component in some breeds. Miniature Poodles, Dachshunds, Yorkshire Terriers and Whippets are predisposed, and nearly 100 percent of Cavalier King Charles Spaniels develop MMVD (Häggström et al., 1995; Parker and Kilroy-Glynn, 2012). MMVD involves progressive degeneration of the mitral valve and in 30 percent of cases, the tricuspid valve too (Fox, 2012). This can lead to an enlarged left atrium and ventricle, reduced cardiac efficiency and the risk of congestive heart failure (CHF).
Approximately 30 percent of patients will develop CHF (Boswood et al., 2017), which carries a much poorer prognosis and diminishes quality of life. Thus, interventions that could effectively slow the progression of MMVD in dogs during the early stages of heart disease, and help delay the onset of CHF, are desirable. Alongside medications such as pimobendan, nutrition may now play a key role in supporting heart health much earlier than previously thought.
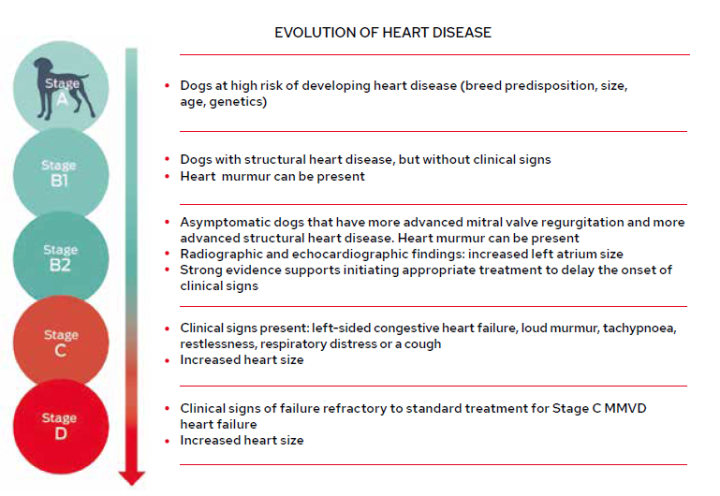
The consensus guidelines of the American College of Veterinary Internal Medicine (ACVIM) classify dogs with MMVD into one of four stages based on clinical findings and echocardiographic evaluation (Figure 1), and recommends appropriate treatment and management strategies dependent on the stage (Keene et al., 2019). This is a useful reference tool for clinicians in general practice.
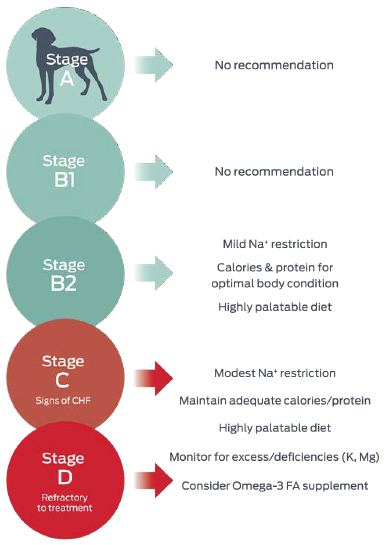
Within the ACVIM consensus guidelines, advice with respect to nutrition primarily targets the later stages of disease, after heart failure occurs, where it is focused on helping ameliorate clinical signs (Figure 2).
Calorie intake in canine cardiac disease
Cardiac cachexia is common in dogs with CHF and is associated with significantly shorter survival times (Freeman, 2009); therefore, ensuring adequate energy intake is crucial. A maintenance calorie intake in Stage C of approximately 60kcal/kg body weight is suggested (Keene et al., 2019). Diet palatability is an important nutritional factor and this, alongside gentle heating of food to increase aroma or offering both wet and dry textures, may help to maximise energy intake.
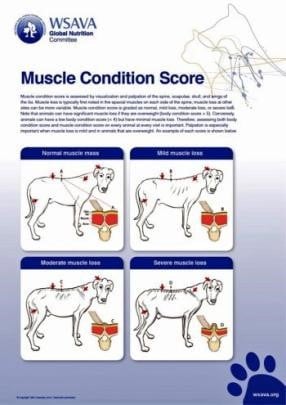
Ensuring adequate protein intake is also key: food with quality protein making up 25 percent of calories should minimise loss of lean body mass (Laflamme, 2005). Clinicians are encouraged to monitor and record both weight and muscle condition score (Figure 3) regularly in patients.
Role of sodium in canine heart disease
Studies in dogs have not shown that sodium has a role in causing heart disease in dogs. However, after heart disease develops, and in particular in heart failure, moderate sodium restriction can help manage symptoms of fluid overload. Reduced cardiac output in CHF stimulates the renin–angiotensin–aldosterone system (RAAS) and leads to increased fluid retention.
Reducing dietary sodium can be helpful to reduce blood volume and heart size in dogs with heart failure (Rush et al., 2000). However, excessive restriction of sodium should be avoided as it stimulates aldosterone activation, which can have adverse effects including promoting inflammation and oxidative stress, and very low sodium levels can induce electrolyte abnormalities.
Reducing dietary sodium can be helpful to reduce blood volume and heart size in dogs with heart failure ... However, excessive restriction of sodium should be avoided as it stimulates aldosterone activation
Several studies in recent years have indicated that excessive restriction of sodium is detrimental in heart failure, causing an increase in mortality in human patients and upregulating RAAS in dogs and cats (Suematsu et al., 2010; Miller et al., 2014). Therefore, avoiding excess or excessive restriction of sodium seems a reasonable approach, although further research is needed to define “excess” (Box 1).
In practical terms, clinicians should consider the sodium content of not only the main meal being fed to a patient with MMVD, but also treats and extras. Some human foods commonly given as treats can have high sodium contents and should be avoided.
There are currently no controlled clinical trials to determine optimum dietary sodium (Na) levels but it appears that:
|
The role of omega-3 in canine cardiac disease
The long-chain omega-3 fatty acids, especially eicosapentaenoic acid (EPA), have demonstrated numerous cardiac benefits. Studies show that omega-3s from fish oil help reduce inflammatory mediators and oxidative stress, stabilise cardiac arrhythmias in dogs, reduce blood pressure and reduce cardiac remodelling (Freeman et al., 1998; Freeman, 2010; Smith et al., 2007). Omega-3 fatty acids may also be important in helping to minimise cachexia (Freeman et al., 2006).
Omega-3 fatty acid supplementation is currently recommended in the ACVIM guidelines from Stage C, but recent studies have suggested they may have a role in dogs with pre-clinical MMVD to help delay disease progression (Li et al., 2019). This is exciting given strategies to delay onset of CHF are desirable.
Recent studies have suggested [omega-3 fatty acid supplementation] may have a role in dogs with pre-clinical MMVD to help delay disease progression
The role of magnesium in canine cardiac disease
The ACVIM guidelines recommend monitoring for magnesium deficiencies and supplementing, where they are identified, from Stage C. Among its roles, magnesium provides antiarrhythmic action, supports mitochondrial function and acts as an antioxidant. In people, inadequate levels of magnesium correlate with heart failure and increased risk for cardiovascular disorders. In heart cells, it complexes with ATP to deliver this molecular energy outside the mitochondria (Del Gobbo et al., 2013; Freeman et al., 2006; Qu et al., 2013). Magnesium is thus a mineral proven to play multiple roles in maintaining healthy heart function. A recent study has suggested it may be helpful to start supplementation earlier in the course of disease as magnesium may have a role to play in delaying progression of disease in dogs with Stage B MMVD (Li et al., 2019).
Other nutrients
In addition to the above nutrients, recent studies have identified several other nutrients that may have a key role to play in supporting dogs with MMVD and, importantly, helping from the early, pre-clinical stages to minimise the likelihood of dogs progressing to Stage C. These include medium-chain triglycerides, amino acids, including taurine, lysine and methionine, and antioxidants (Li et al., 2019).
Medium-chain triglycerides
In heart disease, compromised energy metabolism is a critical factor, and energy production becomes less efficient in dogs with MMVD – in both the pre-clinical and clinical stages (Li et al., 2015). Mitochondria are key for energy production, and in the healthy heart over 70 percent of energy comes from long-chain fatty acid oxidation (Lopaschuk et al., 2010). When needed, mitochondria can use other energy sources – ketones, glucose or branched-chain amino acids – to meet ATP demands but these are less efficient than fatty acids as sources to produce ATP. Diseased hearts can have 30 percent less ATP compared to healthy hearts (Doesnt et al., 2013).

Medium-chain triglycerides (MCTs) have been identified as an alternative energy source for the heart. They are broken down into medium-chain fatty acids (MCFAs) in the liver, which are delivered more rapidly to the mitochondria for energy production (Figure 4). They bypass more complex metabolic pathways and, unlike long-chain fatty acids, do not need carnitine to act as a transporter to enable entry to the mitochondria. Included as part of a dietary “cardiac protection blend” in one study at a level of 5.5 percent, they have been demonstrated to help slow or reverse disease progression in dogs with pre-clinical MMVD (Li et al., 2019). Future studies may explore their role in dogs with clinical signs of heart failure too.
Carnitine, lysine and methionine
Carnitine is key to help transport long-chain fatty acids into the cardiac mitochondria for energy production. The amino acids lysine and methionine are precursors of carnitine, and dietary supplementation with these may be effective in supporting fatty acid oxidation and helping delay MMVD progression (Li et al., 2019). Methionine also functions as an antioxidant and a precursor to taurine.
Taurine and antioxidants
Taurine has a key role in maintaining heart muscle contractility. Deficiencies are linked to the development of heart disease, with associations between low taurine levels and a decrease in sensitivity of cardiac muscle to calcium and the loss of myofibrils (Lake, 1993; Sanderson, 2006). It also acts as an antioxidant. Antioxidants play a key role in reducing oxidative stress and thus minimise potential cell membrane and DNA damage, which may be heightened in the disease state.
Antioxidants play a key role in reducing oxidative stress and thus minimise potential cell membrane and DNA damage, which may be heightened in the disease state
Taurine and additional antioxidants in the form of vitamin E also formed part of the aforementioned “cardiac protection blend” which, in a double-blinded placebo-controlled trial, demonstrated efficacy in delaying progression of MMVD in dogs with Stage B of the disease, including reductions in left atrial diameter and mitral regurgitation (Li et al., 2019). These nutrients have now been incorporated as a cardiac nutritional blend into a veterinary diet available in the UK.
Conclusion
In summary, alongside medical management, nutrition has an important role to play in supporting dogs with MMVD. While nutritional recommendations have traditionally focused primarily on dogs with CHF, recent research suggests more can be done in the earlier stages of disease and may be important in helping to delay progression to clinical signs. This is an exciting and ongoing area of research.
References (click to expand)
Ellie Groves, BA (Hons), VetMB, PCertSAN, MRCVS, was the Veterinary Technical Manager at Purina Petcare until May 2023, and has since moved back to work in small animal clinical practice. Ellie has a passion for nutrition, and her mission is to achieve a greater understanding of clinical nutrition in veterinary professionals, as well as apply that in her day to day role.